
Chemistry, 18.10.2019 01:10 jellybellyje
Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more soluble in kerosene because the non polar structure of kerosene allows the smaller lons to infiltrate the structure of the larger compound and therefore dissolve. nacl will be more soluble in kerosene because kerosene can dissolve all compounds. nacl will be equally soluble in both solvent. nal will be more soluble in water, because water is polar and can separate the cation and anion in the lonic compound, creating very strong ion dipole attractions

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more...
Questions

Business, 11.11.2020 21:40





English, 11.11.2020 21:40

English, 11.11.2020 21:40


English, 11.11.2020 21:40

Social Studies, 11.11.2020 21:40



Mathematics, 11.11.2020 21:40

History, 11.11.2020 21:40




Mathematics, 11.11.2020 21:40


Mathematics, 11.11.2020 21:40

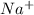 and
and 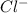 . As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
. As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.

