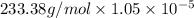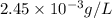Chemistry, 18.10.2019 01:10 naysia2006
Question 6 the mineral barite, (baso.) has a ke of 1.1 x 10" at 25°c. calculate the solubility of barium sulfate in water, in: 6.1. moles per liter 6.2. grams per liter

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Question 6 the mineral barite, (baso.) has a ke of 1.1 x 10" at 25°c. calculate the solubility of ba...
Questions


Mathematics, 31.01.2021 22:20

Arts, 31.01.2021 22:20


Mathematics, 31.01.2021 22:20



Chemistry, 31.01.2021 22:20

English, 31.01.2021 22:20

Mathematics, 31.01.2021 22:20

Mathematics, 31.01.2021 22:20


English, 31.01.2021 22:20


Mathematics, 31.01.2021 22:20

Computers and Technology, 31.01.2021 22:20


Physics, 31.01.2021 22:20


Arts, 31.01.2021 22:20

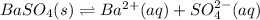
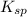 as
as 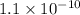 and let the solubility of each specie involved in this reaction is "s". The expression for
and let the solubility of each specie involved in this reaction is "s". The expression for ![K_{sp} = [Ba^{2+}][SO^{-}_{2}]](/tpl/images/0330/0217/72018.png) (Solids are nor considered)
(Solids are nor considered)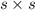
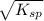
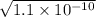
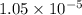
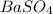 is 233.38 g/mol
is 233.38 g/mol