
Chemistry, 17.10.2019 18:30 lalalaaldgydyhg8823
Determine the [oh−] , ph, and poh of a solution with a [h+] of 2.2×10−12 m at 25 °c. [oh−]= m ph= poh= determine the [h+] , ph, and poh of a solution with an [oh−] of 9.7×10−5 m at 25 °c. [h+]= m ph= poh= determine the [h+] , [oh−] , and poh of a solution with a ph of 10.30 at 25 °c. [h+]= m [oh−]= m poh= determine the [h+] , [oh−] , and ph of a solution with a poh of 9.06 at 25 °c. [h+]= m [oh−]= m ph=

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
Determine the [oh−] , ph, and poh of a solution with a [h+] of 2.2×10−12 m at 25 °c. [oh−]= m ph= po...
Questions

English, 26.09.2021 21:10


Mathematics, 26.09.2021 21:10



Mathematics, 26.09.2021 21:10

Chemistry, 26.09.2021 21:10



Mathematics, 26.09.2021 21:10


French, 26.09.2021 21:10


Geography, 26.09.2021 21:10

English, 26.09.2021 21:10

Law, 26.09.2021 21:10

Mathematics, 26.09.2021 21:10



SAT, 26.09.2021 21:10

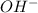 , pH and pOH of the solution is
, pH and pOH of the solution is 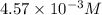 , 11.66 and 2.36 respectively.
, 11.66 and 2.36 respectively.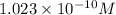 , 4.01 and 9.99 respectively.
, 4.01 and 9.99 respectively.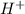 ,
, 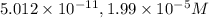 and 3.7 respectively.
and 3.7 respectively.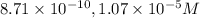 and 4.97 respectively.
and 4.97 respectively.![pH=-\log[H^+]](/tpl/images/0329/0431/cf945.png) .....(1)
.....(1)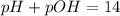 .....(2)
.....(2)![[OH^]](/tpl/images/0329/0431/ffd27.png) of the solution, we use the equation:
of the solution, we use the equation:![pOH=-\log[OH^-]](/tpl/images/0329/0431/fe336.png) ......(3)
......(3)![[H^+]=2.2\times 10^{-12}M](/tpl/images/0329/0431/bd40e.png)
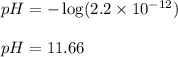
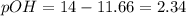
![2.36=-\log[OH^-]](/tpl/images/0329/0431/c7189.png)
![[OH^-]=10^{-2.36}=4.57\times 10^{-5}M](/tpl/images/0329/0431/7f1bf.png)
![[H^+]=9.7\times 10^{-5}M](/tpl/images/0329/0431/f8e3a.png)
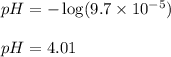
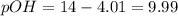
![9.99=-\log[OH^-]](/tpl/images/0329/0431/2903e.png)
![[OH^-]=10^{-9.99}=1.023\times 10^{-10}M](/tpl/images/0329/0431/9422a.png)
![10.30=-\log[H^+]](/tpl/images/0329/0431/05afc.png)
![[H^+]=10^{-10.30)=5.012\times 10^{-11}M](/tpl/images/0329/0431/9890d.png)
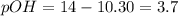
![3.7=-\log[OH^-]](/tpl/images/0329/0431/1954f.png)
![[OH^-]=10^{-3.7}=1.99\times 10^{-4}M](/tpl/images/0329/0431/a2180.png)
![9.03=-\log[H^+]](/tpl/images/0329/0431/15752.png)
![[H^+]=10^{-9.30)=8.71\times 10^{-10}M](/tpl/images/0329/0431/070a8.png)
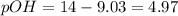
![4.97=-\log[OH^-]](/tpl/images/0329/0431/c7d38.png)
![[OH^-]=10^{-4.97}=1.07\times 10^{-5}M](/tpl/images/0329/0431/10104.png)



