
Chemistry, 17.10.2019 19:00 unknown235
Which one of the following statements is true?
a. the enthalpy change for a reaction is independent of the state of the reactants and products.
b. enthalpy is an intensive property.
c. enthalpy is a state function.
d. h is the value of q measured under conditions of constant volume.
e. the enthalpy change of a reaction is the reciprocal of the δh of the reverse reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Which one of the following statements is true?
a. the enthalpy change for a reaction is inde...
a. the enthalpy change for a reaction is inde...
Questions

Advanced Placement (AP), 07.06.2021 18:50



Mathematics, 07.06.2021 18:50



Spanish, 07.06.2021 18:50

Mathematics, 07.06.2021 18:50

Mathematics, 07.06.2021 18:50

Mathematics, 07.06.2021 18:50


History, 07.06.2021 18:50


Mathematics, 07.06.2021 18:50




Physics, 07.06.2021 18:50


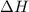 and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.
and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.

