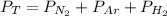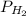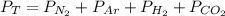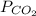Chemistry, 17.10.2019 18:10 shaylasimonds587
Dalton’s law calculationa mixture of h₂, n₂ and ar gases is present in a steel cylinder. the total pressure within the cylinder is 675 mm hg and the partial pressures of n₂ and ar are, respectively, 354 mm hg and 235 mm hg. if co₂ gas is added to the mixture, at constant temperature, until the total pressure reaches 842 mm hg, what is the partial pressure, in mm hg, of the following? a) co₂, 167 b) n₂, 354 c) ar, 235 d) h₂, 8629

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Dalton’s law calculationa mixture of h₂, n₂ and ar gases is present in a steel cylinder. the total...
Questions

Health, 29.08.2019 21:30

Computers and Technology, 29.08.2019 21:30

Mathematics, 29.08.2019 21:30







Mathematics, 29.08.2019 21:30

History, 29.08.2019 21:30

History, 29.08.2019 21:30



Computers and Technology, 29.08.2019 21:30


Geography, 29.08.2019 21:30

History, 29.08.2019 21:30

English, 29.08.2019 21:30

Mathematics, 29.08.2019 21:30

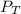 = total presure of the system (675 mm Hg).
= total presure of the system (675 mm Hg).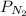 = partial pressure of N₂ (354 mm Hg).
= partial pressure of N₂ (354 mm Hg).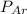 = partial pressure of Ar (235 mm Hg).
= partial pressure of Ar (235 mm Hg).