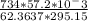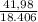At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. a 0.8765-g sample of impure sodium chlorate was heated until the production of oxygen gas ceased. the oxygen gas collected over water occupied 57.2 ml at a temperature of 22ºc and a pressure of 734 torr. calculate the mass percent of nahco₃ in the original sample. (at 22ºc the vapor pressure of water is 19.8 torr.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

You know the right answer?
At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. a 0....
Questions

Chemistry, 19.05.2021 02:30

History, 19.05.2021 02:30


English, 19.05.2021 02:30

Mathematics, 19.05.2021 02:30


Mathematics, 19.05.2021 02:40

Mathematics, 19.05.2021 02:40

Mathematics, 19.05.2021 02:40

English, 19.05.2021 02:40

English, 19.05.2021 02:40

Advanced Placement (AP), 19.05.2021 02:40

Business, 19.05.2021 02:40

Chemistry, 19.05.2021 02:40

Arts, 19.05.2021 02:40


Mathematics, 19.05.2021 02:40

English, 19.05.2021 02:40

History, 19.05.2021 02:40

Mathematics, 19.05.2021 02:40