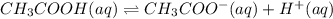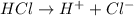Choose the statement below that is true.
a. a weak acid solution consists of mostly non-ioniz...

Chemistry, 17.10.2019 17:30 happy121906
Choose the statement below that is true.
a. a weak acid solution consists of mostly non-ionized acid molecules.
b. the term "strong electrolyte" means that the substance is extremely reactive.
c. a strong acid solution consists of only partially ionized acid molecules.
d. the term "weak electrolyte" means that the substance is inert.
e. a molecular compound that does not ionize in solution is considered a strong electrolyte.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Questions

English, 22.09.2019 21:30





History, 22.09.2019 21:30


Biology, 22.09.2019 21:30

Mathematics, 22.09.2019 21:30







History, 22.09.2019 21:30


Mathematics, 22.09.2019 21:30

Health, 22.09.2019 21:30

English, 22.09.2019 21:30