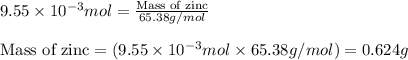Small quantites of hydrogen gas can be prepared in the laboratory by the addition of aqueous hydrochloric acid to metallic zinc. typically, the hydrogen gas is bubbled through water for collection and becomes saturated with water vapor. suppose 240. ml of hydrogen gas is collected at 30. c and has a total pressure of 1.032 atm by this process. what is the partial pressure of hydrogen gas in the sample? how many grams of zinc must have reacted to produce this quantity of hydrogen? (the vapor pressure of water is 32 torr at 30 c).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 23.06.2019 11:30
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1
You know the right answer?
Small quantites of hydrogen gas can be prepared in the laboratory by the addition of aqueous hydroch...
Questions

Mathematics, 11.02.2021 20:30

Mathematics, 11.02.2021 20:30




Computers and Technology, 11.02.2021 20:30

Computers and Technology, 11.02.2021 20:30

Mathematics, 11.02.2021 20:30


History, 11.02.2021 20:30



Social Studies, 11.02.2021 20:30



Physics, 11.02.2021 20:30

Computers and Technology, 11.02.2021 20:30

Mathematics, 11.02.2021 20:30

Mathematics, 11.02.2021 20:30

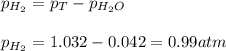
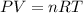
![30^oC=[30+273]K=303K](/tpl/images/0328/9260/fd4b3.png)
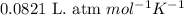
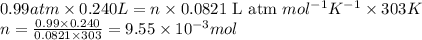
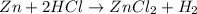
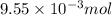 of hydrogen gas is produced from =
of hydrogen gas is produced from =  of zinc metal
of zinc metal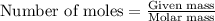
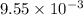 moles
moles