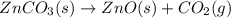The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 71.5 kj of heat per mole of znco3. you may want to reference (pages 177 - 178) section 5.4 while completing this problem. part apart complete write a balanced equation for the reaction. express your answer as a chemical equation. identify all of the phases in your answer. znco3(s)→zno(s)+co2(g) previous answers correct the reactant and products of the decomposition reaction are given in the introduction. znco3(s) is broken down into zno(s) and co2(g). part b what is the enthalpy of the reaction above?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1


Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2
You know the right answer?
The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 7...
Questions







History, 14.12.2021 20:30




Mathematics, 14.12.2021 20:40

Mathematics, 14.12.2021 20:40

English, 14.12.2021 20:40



Social Studies, 14.12.2021 20:40

English, 14.12.2021 20:40


Physics, 14.12.2021 20:40