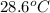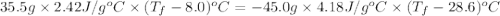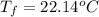Chemistry, 17.10.2019 06:00 steven2996
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (density = 1.0 g/ml) initially at 28.6 ∘c in an insulated beaker, what is the final temperature of the mixture, assuming that no heat is lost? (cetoh=2.42j/(g⋅∘

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (den...
Questions


Mathematics, 10.12.2020 02:10







Mathematics, 10.12.2020 02:10



Mathematics, 10.12.2020 02:10


Mathematics, 10.12.2020 02:10

English, 10.12.2020 02:10


Biology, 10.12.2020 02:10

Business, 10.12.2020 02:10

History, 10.12.2020 02:10

Mathematics, 10.12.2020 02:10

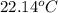
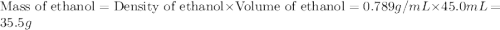
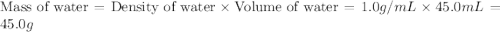
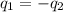
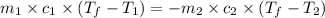
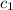 = specific heat of ethanol =
= specific heat of ethanol = 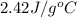
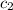 = specific heat of water =
= specific heat of water = 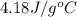
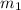 = mass of ethanol = 35.5 g
= mass of ethanol = 35.5 g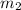 = mass of water = 45.0 g
= mass of water = 45.0 g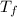 = final temperature of mixture = ?
= final temperature of mixture = ?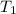 = initial temperature of ethanol =
= initial temperature of ethanol = 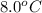
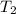 = initial temperature of water =
= initial temperature of water =