
Chemistry, 17.10.2019 05:00 trinityanne1738
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.200 m and [no]=0.600 m. n2(g)+o2(g)↽−−⇀2no(g) if more no is added, bringing its concentration to 0.900 m, what will the final concentration of no be after equilibrium is re‑established?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.200 m and [no]=0.600...
Questions




















Chemistry, 17.09.2019 23:00

![K_{eq}=\frac{[NO]^2_{eq}}{[N_2]_{eq}[O_2]_{eq}} \\K_{eq}=\frac{0.6^2}{0.2*0.2}\\ K_{eq}=9](/tpl/images/0327/3782/fd314.png)
![9=\frac{[NO]^2_{eq}}{[N_2]_{eq}[O_2]_{eq}}\\9=\frac{[0.9+2x]^2}{[0.2-x][0.2-x]}](/tpl/images/0327/3782/c867b.png)
![\frac{1}{9} =\frac{[N_2]_{eq}[O_2]_{eq}}{[NO]^2_{eq}}\\\frac{1}{9} =\frac{[0.2+x][0.2+x]}{[0.9-2x]^2}](/tpl/images/0327/3782/3755f.png)
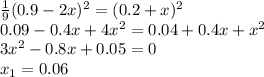
![[NO]_{eq}=0.9-0.06=0.84M](/tpl/images/0327/3782/19d1e.png)


