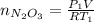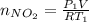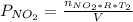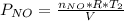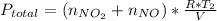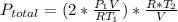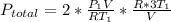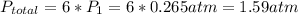Acontainer of n2o3(g) has a pressure of 0.265 atm. when the absolute temperature of the n2o3(g) is tripled, the gas completely decomposes, producing no2(g) and no(g). calculate the final pressure of the gas mixture, assuming that the container volume does not change.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Acontainer of n2o3(g) has a pressure of 0.265 atm. when the absolute temperature of the n2o3(g) is t...
Questions


Social Studies, 11.03.2020 18:38













Chemistry, 11.03.2020 18:38

Spanish, 11.03.2020 18:38

French, 11.03.2020 18:38



Mathematics, 11.03.2020 18:38

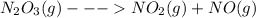
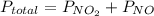
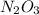 because it decomposed completely.
because it decomposed completely.