
Chemistry, 16.10.2019 22:00 nessuhbae6731
Suppose you have three monodisperse polymer samples, a, b, and c, with molar masses of 100,000 g/mol, 200,000 g/mol, and 300,000 g/mol, respectively. calculate the number average and weight-average molar mass of a blend obtained by mixing a, b, and c as follows: a) in equal proportions by weight b) in the molar ratio 1: 1: 1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

You know the right answer?
Suppose you have three monodisperse polymer samples, a, b, and c, with molar masses of 100,000 g/mol...
Questions

Mathematics, 12.02.2021 02:30

Mathematics, 12.02.2021 02:30

Biology, 12.02.2021 02:30

English, 12.02.2021 02:30



Mathematics, 12.02.2021 02:30





History, 12.02.2021 02:30




Mathematics, 12.02.2021 02:30

Mathematics, 12.02.2021 02:30



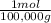 = 1x10⁻⁵ moles A
= 1x10⁻⁵ moles A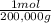 = 5x10⁻⁶ moles B
= 5x10⁻⁶ moles B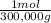 = 3,33x10⁻⁶ moles C
= 3,33x10⁻⁶ moles C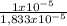 ×100 = 54,5%
×100 = 54,5%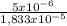 ×100 = 27,3%
×100 = 27,3%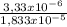 ×100 = 18,2%
×100 = 18,2%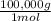 = 100,000g A
= 100,000g A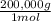 = 200,000g B
= 200,000g B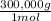 = 300,000g C
= 300,000g C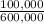 ×100 = 16,7%
×100 = 16,7%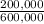 ×100 = 33,3%
×100 = 33,3%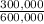 ×100 = 50,0%
×100 = 50,0%

