
Chemistry, 16.10.2019 21:20 sdwhitneyhillis
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one reactant a is given by: = k [aj? -d[a/dt where k is the reaction rate coefficient. derive an expression for the half-life of a in terms of k and the concentration of a at time t-0 (ao)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one rea...
Questions

Chemistry, 06.01.2021 20:40



Biology, 06.01.2021 20:40

Mathematics, 06.01.2021 20:40


Biology, 06.01.2021 20:40



Mathematics, 06.01.2021 20:40

Mathematics, 06.01.2021 20:40

English, 06.01.2021 20:40

History, 06.01.2021 20:40



Mathematics, 06.01.2021 20:40

Computers and Technology, 06.01.2021 20:40

Mathematics, 06.01.2021 20:40


Mathematics, 06.01.2021 20:40

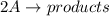
![\textrm{rate}=-\dfrac{\Delta[\textrm A]}{2\Delta t}=k[\textrm A]^2](/tpl/images/0326/2107/66a24.png)
![\dfrac{d[A]}{dt}=-k[A]^2](/tpl/images/0326/2107/6f64e.png)
![\int_{[A_t]}^{[A_0]}\frac{d[A]}{[A]^2}=-\int_{0}^{t}kdt](/tpl/images/0326/2107/7af1b.png)
![\dfrac{1}{[A]} = \dfrac{1}{[A]_0}+kt](/tpl/images/0326/2107/704d4.png)
![[A_t]](/tpl/images/0326/2107/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0326/2107/9a686.png) is the initial concentration
is the initial concentration![[A_t]=\frac{1}{2}\times [A_0]](/tpl/images/0326/2107/09fb2.png)
![t_{1/2}=\dfrac{1}{k[A_o]}](/tpl/images/0326/2107/4b6c5.png)


