Explanation:
Problem 1:
Plants utilize light energy in the photosynthesis process to synthesize glucose, C₆H₁₂O₆, from CO₂ and H₂O by way of the reaction 6CO₂ +6H₂O → C₆H₁₂O₆ +60₂. How many grams of CO₂ are consumed in the production of 90 g of glucose?
Given parameters:
Mass of glucose = 10g
Unkown:
Mass of CO₂ consumed = ?
Solution:
The reaction equation is given as:
6CO₂ + 6H₂O → C₆H₁₂O₆ +60₂
To solve this problem, the mole concept is a good approach. First work from the known to the unkown. The known is the given parameter which is the mass of glucose. Using the mass of glucose, estimate its number of moles and compare to that of the unknown carbon dioxide gas. Then find the mass from the obtained number of moles.
##
Number of moles of C₆H₁₂O₆ = 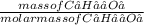
given that atomic weight of:
C = 12.01g/mol
H = 1.01g/mol
O = 16.00g/mol
Molar mass of C₆H₁₂O₆ = (6 x 12.01) + (12 x 1.01) + (6 x 16) = 180g/mol
Number of moles = 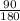 = 0.5mol
= 0.5mol
##
from the reaction equation,
1 mole of glucose is formed from 6 moles of CO₂
0.5 mole of glucose would be formed from (6 x 0.5)mole = 3moles
##
mass of CO₂ = number of moles x molar mass
molar mass of CO₂ = 12 + 2(16) = 44g/mol
mass of CO₂ = 3 x 44 = 132g of CO₂
--------------------------------------------------------------------------------------------------------------
Problem 2:
For the reaction of 100.0 g of NaOH with Cl₂, 2NaOH + Cl₂ → NaClO + NaCI +H₂O, give the masses of each of the products.
Given parameters:
Mass of NaOH = 100g
Unknown:
mass of NaClo = ?
mass of NaCl = ?
mass of H₂O = ?
Solution:
Use the same procedure as highlighted in problem 1:
equation of reaction:
2NaOH + Cl₂ → NaClO + NaCI +H₂O
##
Number of moles of NaOH = 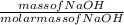
Molar mass of NaOH:
Atomic weight of Na = 23g/mol
Atomic weight of O = 16g/mol
Atomic weight of H = 1g/mol
Molar mass of NaOH = 23 + 16 + 1 = 40g/mol
Number of moles of NaOH = 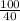 = 2.5mole
= 2.5mole
##
From the equation;
first product NaClO:
2 moles of NaOH produced 1 mole of NaClO
2.5 moles of NaOH will produce 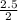 mole = 1.25mole of NaClO
mole = 1.25mole of NaClO
mass of NaClO = number of moles x molar mass
molar mass of NaClO = 23 + 35.5 + 16 = 74.5g/mol
mass of NaClO = 1.25 x 74.5 = 93.13g of NaClO
Second product NaCl:
2 moles of NaOH produced 1 mole of NaCl
2.5 mole of NaOH will produce 1.25 mole of NaCl
mass of NaCl = number of moles x molar mass
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
mass of NaCl = 1.25 x 58.5 = 73.13g of NaCl
Third product H₂O:
2 moles of H₂O produced 1 mole of H₂O
2.5 mole of H₂O will produce 1.25 mole of H₂O
mass of H₂O = number of moles x molar mass
molar mass of H₂O = 2(1) + 16 = 18g/mol
mass of H₂O = 1.25 x 18 = 22.5g of H₂O
--------------------------------------------------------------------------------------------------------------
Hydrochloric acid (HCl gas dissolved in water) reacts with calcium carbonate in piece of limestone as follows: CaCO₃ + 2HCI → CaCl₂ + CO₂ + H₂0. If 14.6 g of CO₂ are produced in this reaction, what is the total mass of reactants and the total mass of the products?
Given parameters:
Mass of CO₂ = 14.6g
Unkown:
Total mass of products = ?
Total mass of reactants =?
Solution:
Total mass of products = mass of CaCl₂ + mass of CO₂ + mass of H₂O
Total mas of reactants = mass of CaCO₃ + mass of HCl
Using the procedures highlighted in the first problem, solve for the number of moles of the known:
##
Number of moles of CO₂ = 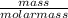
molar mass of CO₂ = 12 + 2(16) = 44g/mol
Number of moles of CO₂ = 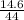 = 0.33mole
= 0.33mole
##
number of moles of all species:
These species are in a ratio of one to one and the will have the same number of moles as that of the known:
Number of moles of CaCl₂ = 0.33mole
Number of moles of H₂O = 0.33mole
Number of moles of CaCO₃ = 0.33mole
For HCl:
1 mole of CO₂ is produced from 2 moles of HCl
0.33 mole of CO₂ will produce 2 x 0.33moles = 0.66moles of HCl
##
Mass of each species:
Mass = number of moles x molar mass
molar mass of CaCl₂ = 40 + 2(35.5) = 111g/mol
molar mass of H₂O = 2(1 ) + 16 = 18g/mol
molar mass of CaCO₃ = 40 + 12 + 3(16) = 100g/mol
molar mass of HCl = 1 + 35.5 = 36.5g/mol
mass of CaCl₂ = 0.33 x 111 = 36.83g
mass of H₂O = 0.33 x 18 = 5.94g
mass of CaCO₃ = 0.33 x 100 = 33g
mass of HCl = 0.66 x 36.5 = 24.09g
##
Total mass of reactants = 33g + 24.09g = 57.1g
Total mass of products = 36.83g + 14.6g + 5.94g = 57.3g





























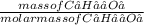
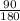 = 0.5mol
= 0.5mol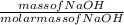
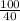 = 2.5mole
= 2.5mole 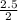 mole = 1.25mole of NaClO
mole = 1.25mole of NaClO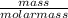
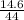 = 0.33mole
= 0.33mole

