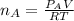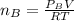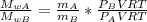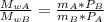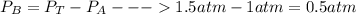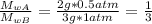Chemistry, 16.10.2019 04:30 atsuedem974
When 2 g of gaseous substance a are introduced into an initially evacuated flask kept at 25°c, the pressure is found to be 1 atm. three grams of gaseous substance b are then added to the 2g of a, and the pressure is now found to be 1.5 atm. assuming ideal gas behavior. calculating the ratio of molecular weights, that is, mwa /mwb

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
When 2 g of gaseous substance a are introduced into an initially evacuated flask kept at 25°c, the p...
Questions

History, 11.03.2021 01:10



Mathematics, 11.03.2021 01:10

Mathematics, 11.03.2021 01:10




Mathematics, 11.03.2021 01:10

Mathematics, 11.03.2021 01:10




History, 11.03.2021 01:10

Mathematics, 11.03.2021 01:10




Chemistry, 11.03.2021 01:10

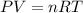
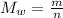
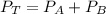
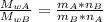 (equation 1)
(equation 1)