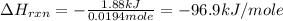Chemistry, 16.10.2019 03:30 kealinwiley
You place 36.5 ml of 0.266 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 56.6 ml of 0.648 m hcl, also at 25.00°c. after stirring, the final temperature is 29.83°c. {assume that the total volume is the sum of the individual volumes and that the final solution has the same density (1.00 g/ml) and specific heat capacity (4.184 j/g°c) as water}. calculate the change in enthalpy, δh, of the reaction (in kj/mol) of water formed. enter the appropriate sign (+/

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
You place 36.5 ml of 0.266 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 56.6 ml of 0.648...
Questions

English, 18.03.2021 02:50

Chemistry, 18.03.2021 02:50


History, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50


English, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50



Mathematics, 18.03.2021 02:50

Arts, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

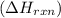 is, -96.9 kJ/mole
is, -96.9 kJ/mole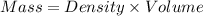
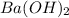
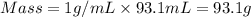
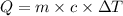
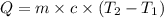
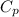 = specific heat capacity of water =
= specific heat capacity of water = 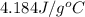
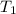 = initial temperature =
= initial temperature = 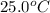
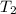 = final temperature =
= final temperature = 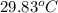
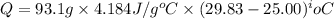
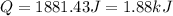 (1 kJ = 1000 J)
(1 kJ = 1000 J)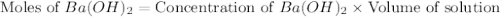
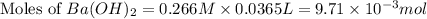
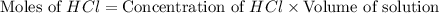
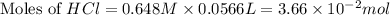
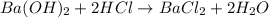
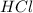
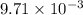 moles of
moles of 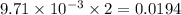 moles of
moles of 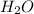
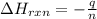
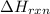 = enthalpy of reaction = ?
= enthalpy of reaction = ?