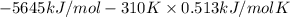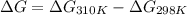Chemistry, 16.10.2019 04:00 Fatimaneedhelp
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gibbs energy is -5798 kj/mol. assume ∆h does not change to estimate the additional non-expansion work that may be obtained by raising the temperature to blood temperature, 37o c. enter your answer in kj/mol to two significant figures and do not enter the units.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gi...
Questions

Mathematics, 07.07.2021 17:20

Mathematics, 07.07.2021 17:20





Chemistry, 07.07.2021 17:20

History, 07.07.2021 17:20

Biology, 07.07.2021 17:20

Mathematics, 07.07.2021 17:20

Mathematics, 07.07.2021 17:20

English, 07.07.2021 17:20

Mathematics, 07.07.2021 17:20



Biology, 07.07.2021 17:20

Medicine, 07.07.2021 17:20



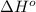 = -5645 kJ/mol
= -5645 kJ/mol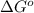 = -5798 kJ/mol
= -5798 kJ/mol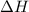 and
and 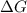 are as follows.
are as follows.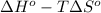
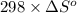
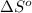 = 0.513 kJ/mol K
= 0.513 kJ/mol K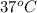 = (37 + 273) K = 310 K
= (37 + 273) K = 310 K