
Naturally occurring silicon has an atomic mass of 28.086 and consists of three isotopes. the major isotope is 28si, natural abundance 92.23%, relative atomic mass 27.97693. the next most abundant isotope is 29si, relative atomic mass 28.97649. the third isotope is 30si whose natural abundance is in the ratio of 0.6592 to that of 29si.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Naturally occurring silicon has an atomic mass of 28.086 and consists of three isotopes. the major i...
Questions

Geography, 07.10.2019 18:30

History, 07.10.2019 18:30

Mathematics, 07.10.2019 18:30




Mathematics, 07.10.2019 18:30


Health, 07.10.2019 18:30

Mathematics, 07.10.2019 18:30

Mathematics, 07.10.2019 18:30

History, 07.10.2019 18:30

Physics, 07.10.2019 18:30

Mathematics, 07.10.2019 18:30




Social Studies, 07.10.2019 18:30

English, 07.10.2019 18:30

Biology, 07.10.2019 18:30

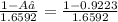 =0.0468
=0.0468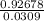 =Si³⁰
=Si³⁰

