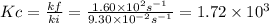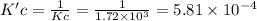At a given temperature, the elementary reaction a > b in the forward direction is first order in a with a rate constant of 1.60*10^2 s^-1. the reverse reaction is first order in b and the rate constant is 9.30*10^-2 s^-1what is the value of the equilibrium constant for the reaction a > b at this temperature? what is the value of equilibrium constant for the reaction b--> a at this temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Compare these two waves : a. the blue wave has a higher pitch, but the orange wave is louder. b.the blue and orange waves have the same volume, but the blue wave has a higher pitch. c.the blue and orange waves have the same pitch, but the blue wave is louder. d.the orange wave has a higher pitch, but the blue wave is louder.
Answers: 1


Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
At a given temperature, the elementary reaction a > b in the forward direction is first order in...
Questions

Mathematics, 14.11.2020 05:30

Mathematics, 14.11.2020 05:30







History, 14.11.2020 05:30

Spanish, 14.11.2020 05:30


Mathematics, 14.11.2020 05:30

English, 14.11.2020 05:30



Mathematics, 14.11.2020 05:30


Mathematics, 14.11.2020 05:30


English, 14.11.2020 05:30