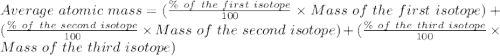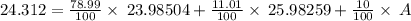Chemistry, 15.10.2019 21:20 francisco42002
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major isotope is mg24, natural abundance 78.99%, relative atomic mass 23.98504. the next most abundant isotope is mg26, relative atomic mass 25.98259. the third most abundant isotope is mg25 whose natural abundance is in the ratio of 0.9083 to that of mg26. find the relative atomic mass of mg25.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major...
Questions



Biology, 15.06.2020 21:57



Mathematics, 15.06.2020 21:57




Mathematics, 15.06.2020 21:57


English, 15.06.2020 21:57

Mathematics, 15.06.2020 21:57



Mathematics, 15.06.2020 21:57




Mathematics, 15.06.2020 21:57