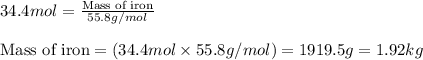Chemistry, 15.10.2019 21:10 jojo887314
Before arc welding was developed, a displacement reaction involving aluminum and iron(iii) oxide was commonly used to produce molten iron (the thermite process). this reaction was used, for example, to connect sections of iron railroad track. calculate the mass of molten iron produced when 2.28 kg of aluminum reacts with 17.2 mol of iron(iii) oxide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Before arc welding was developed, a displacement reaction involving aluminum and iron(iii) oxide was...
Questions

Chemistry, 13.05.2021 05:40

Mathematics, 13.05.2021 05:50





Mathematics, 13.05.2021 05:50

Biology, 13.05.2021 05:50

Physics, 13.05.2021 05:50

Mathematics, 13.05.2021 05:50

Chemistry, 13.05.2021 05:50


Mathematics, 13.05.2021 05:50

Arts, 13.05.2021 05:50

English, 13.05.2021 05:50

Spanish, 13.05.2021 05:50

Mathematics, 13.05.2021 05:50


Chemistry, 13.05.2021 05:50

Spanish, 13.05.2021 05:50

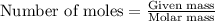 ......(1)
......(1)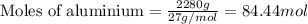
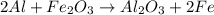
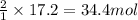 of aluminium
of aluminium