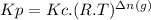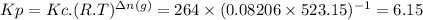Chemistry, 15.10.2019 18:20 thestarlexyp32wpj
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the other phosphorus compounds, coexist in equilibrium through the reactionpcl3(g) + cl2(g) = pcl5(g)at 250 ᵒc , an equilibrium mixture in a 25.0 l flask contains 0.105 g pcl5, 0.220 g pcl3 and 2.12 g of cl2. what are the values of(a) kc(b) kp for this reaction at 250 ᵒc ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the oth...
Questions

English, 12.04.2021 17:40

Mathematics, 12.04.2021 17:40

English, 12.04.2021 17:40


Biology, 12.04.2021 17:40

Business, 12.04.2021 17:40

English, 12.04.2021 17:40

Social Studies, 12.04.2021 17:40

Mathematics, 12.04.2021 17:40

Mathematics, 12.04.2021 17:40




Mathematics, 12.04.2021 17:40



English, 12.04.2021 17:40

Mathematics, 12.04.2021 17:40


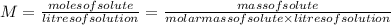
![[PCl_{3}]=\frac{0.220g}{137.5g/mol \times 25.0L } =6.40 \times 10^{-5} M](/tpl/images/0322/4252/44575.png)
![[Cl_{2}]=\frac{2.12g}{71.0g/mol \times 25.0L } =1.19 \times 10^{-3}M](/tpl/images/0322/4252/f9cf8.png)
![[PCl_{5}]=\frac{0.105g}{208.5g/mol \times 25.0L } =2.01 \times 10^{-5} M](/tpl/images/0322/4252/137a9.png)
![Kc=\frac{[PCl_{5}]}{[PCl_{3}]\times [Cl_{2}] } =\frac{2.01 \times 10^{-5} }{6.40 \times 10^{-5} \times 1.19 \times 10^{-3} } =264](/tpl/images/0322/4252/1590c.png)