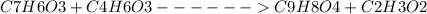Chemistry, 15.10.2019 18:00 kierafisher05
Aspirin can be prepared from salicylic acid ( c 7 h 6 o 3 ), which has a molar mass of 138.12 g/mol, and acetic anhydride ( c 4 h 6 o 3 ), which has a molar mass of 102.04 g/mol. the density of acetic anhydride is 1.082 g/ml. c 7 h 6 o 3 + c 4 h 6 o 3 ⟶ c 9 h 8 o 4 + c 2 h 3 o 2 what is the yield of aspirin ( c 9 h 8 o 4 ), which has a molar mass of 180.15 g/mol, possible when reacting 2.18 g of salicylic acid with 1.01 ml of acetic anhydride?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Aspirin can be prepared from salicylic acid ( c 7 h 6 o 3 ), which has a molar mass of 138.12 g/mol,...
Questions



Mathematics, 02.11.2019 21:31


Mathematics, 02.11.2019 21:31

History, 02.11.2019 21:31



History, 02.11.2019 21:31

Social Studies, 02.11.2019 21:31

Biology, 02.11.2019 21:31

Mathematics, 02.11.2019 21:31



Mathematics, 02.11.2019 21:31

Biology, 02.11.2019 21:31

Computers and Technology, 02.11.2019 21:31


SAT, 02.11.2019 21:31