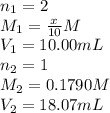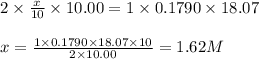Chemistry, 15.10.2019 18:00 kingalex7575
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1790 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrates it with the standard solution. the endpoint was reached after the addition of 18.07 ml of the standard solution. what is the concentration of the original sulfuric acid solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

You know the right answer?
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standa...
Questions

Mathematics, 30.07.2021 01:00

Mathematics, 30.07.2021 01:00



History, 30.07.2021 01:00

Mathematics, 30.07.2021 01:00

Mathematics, 30.07.2021 01:00


Mathematics, 30.07.2021 01:00




Mathematics, 30.07.2021 01:00






Mathematics, 30.07.2021 01:00

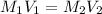
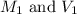 are the molarity and volume of the concentrated sulfuric acid solution
are the molarity and volume of the concentrated sulfuric acid solution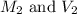 are the molarity and volume of diluted sulfuric acid solution
are the molarity and volume of diluted sulfuric acid solution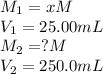
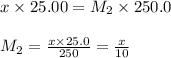
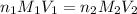
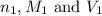 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 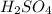
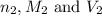 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.