
Chemistry, 15.10.2019 17:20 joelpimentel
In 1897 the swedish explorer andree tried to reach the north pole in a balloon. the balloon was filled with hydrogen gas. the hydrogen gas was prepared from iron splints and diluted sulfuric acid. the reaction is given below. fe(s) + h2so4(aq) feso4(aq) + h2(g)the volume of the balloon was 4870 m3 and the loss of hydrogen gas during filling was estimated at 20.%. what mass of iron splints and 95% (by mass) h2so4 were needed to ensure the complete filling of the balloon? assume a temperature of 0°c, a pressure of 1.0 atm during filling, and 100% yield. iron splints = % sulfuric acid =

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
In 1897 the swedish explorer andree tried to reach the north pole in a balloon. the balloon was fill...
Questions


World Languages, 03.12.2020 14:00

Mathematics, 03.12.2020 14:00


Spanish, 03.12.2020 14:00



Social Studies, 03.12.2020 14:00

Mathematics, 03.12.2020 14:00

World Languages, 03.12.2020 14:00



Mathematics, 03.12.2020 14:00

Mathematics, 03.12.2020 14:00

Mathematics, 03.12.2020 14:00

Physics, 03.12.2020 14:00


Mathematics, 03.12.2020 14:00


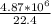 = 2,17 *10^5 moles (if 100% of the H2 is used)
= 2,17 *10^5 moles (if 100% of the H2 is used)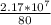 = 2,68*10^5 moles
= 2,68*10^5 moles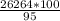 = 27646.32 kg
= 27646.32 kg

