
The decomposition of n2o to n2 and o2 is a first-order reaction. at 730°c, the rate constant of the reaction is 1.94 × 10-4 min-1. if the initial pressure of n2o is 4.70 atm at 730°c, calculate the total gas pressure after one half-life. assume that the volume remains constant. slatter

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
The decomposition of n2o to n2 and o2 is a first-order reaction. at 730°c, the rate constant of the...
Questions



History, 29.09.2019 21:00


Mathematics, 29.09.2019 21:10


Advanced Placement (AP), 29.09.2019 21:10


Mathematics, 29.09.2019 21:10

Mathematics, 29.09.2019 21:10



Biology, 29.09.2019 21:10

Mathematics, 29.09.2019 21:10

Health, 29.09.2019 21:10

Mathematics, 29.09.2019 21:10



History, 29.09.2019 21:10

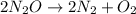
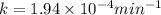
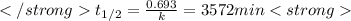
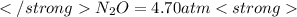
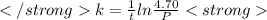
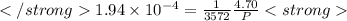
 after first half life = 2.35 = 4.70 - 2x
after first half life = 2.35 = 4.70 - 2x after first half life = 2x = 2(1.175) = 2.35 ATM
after first half life = 2x = 2(1.175) = 2.35 ATM

