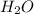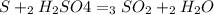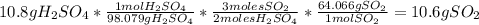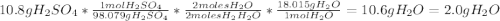Chemistry, 15.10.2019 04:00 johnnyboy41706
For each of the following unbalanced equations, calculate how many grams of each product would be produced by complete reaction of 10.8 g of the second reactant. (a) s(s) + h2so4(aq) → so2(g) + h2o(l) so2 webassign will check your answer for the correct number of significant figures. g h2o webassign will check your answer for the correct number of significant figures. g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
For each of the following unbalanced equations, calculate how many grams of each product would be pr...
Questions


Chemistry, 02.10.2019 23:40

Mathematics, 02.10.2019 23:40

Mathematics, 02.10.2019 23:40



Mathematics, 02.10.2019 23:40




Mathematics, 02.10.2019 23:40

Mathematics, 02.10.2019 23:40



English, 02.10.2019 23:40

English, 02.10.2019 23:40




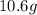 of
of 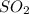
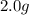 of
of