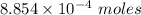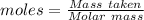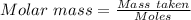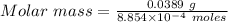Chemistry, 15.10.2019 01:10 nuncasemeoovideo999
During one researcher's experiment, the total mass of the gas-generating solid and entire assembly was 52.1487g before the reaction and 52.1098g after the reaction. if the number of moles of gas generated was calculated to be 8.854 x 10-4 mol, what is the molar mass of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
During one researcher's experiment, the total mass of the gas-generating solid and entire assembly w...
Questions


Social Studies, 21.08.2019 00:00






History, 21.08.2019 00:00

English, 21.08.2019 00:00

Mathematics, 21.08.2019 00:00



Mathematics, 21.08.2019 00:00


Mathematics, 21.08.2019 00:00


Chemistry, 21.08.2019 00:00



Biology, 21.08.2019 00:00