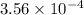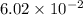Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2 nh_3 (g)
during the production process, the production engineer determinesthe reaction quotient to be q_2 = 3.56ã10â4. ifk_2 = 6.02ã10â2, what can besaid about the reaction?
1)the reaction has reached equilibrium.
2)the reaction is not at equilibrium and willproceed to the left.
3)the reaction is not at equilibrium and willproceed to the right.
4)the reaction is not at equilibrium, but it isnot possible to determine whether the reaction needs to proceedright or left to reach equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


You know the right answer?
Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
Questions


History, 30.09.2019 12:30

Chemistry, 30.09.2019 12:30

Social Studies, 30.09.2019 12:30

History, 30.09.2019 12:30




History, 30.09.2019 12:30

Social Studies, 30.09.2019 12:30


Biology, 30.09.2019 12:30

Chemistry, 30.09.2019 12:30

Chemistry, 30.09.2019 12:30



Arts, 30.09.2019 12:30

Mathematics, 30.09.2019 12:30

Mathematics, 30.09.2019 12:30

Mathematics, 30.09.2019 12:30