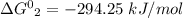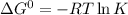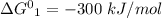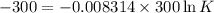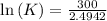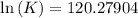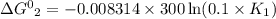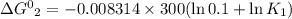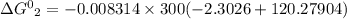Chemistry, 14.10.2019 23:30 kfnldkl1782
One enzyme-catalyzed reaction in a biochemical cycle has an equilibrium constant that is 10 times the equilibrium constant of a second reaction. if the standard gibbs energy of the former reaction is −300 kj mol−1, what is the standard reaction gibbs energy of the second reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3


Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
One enzyme-catalyzed reaction in a biochemical cycle has an equilibrium constant that is 10 times th...
Questions

Social Studies, 04.08.2019 08:30



Mathematics, 04.08.2019 08:30


History, 04.08.2019 08:30

Social Studies, 04.08.2019 08:30


History, 04.08.2019 08:30



Computers and Technology, 04.08.2019 08:30

History, 04.08.2019 08:30

Geography, 04.08.2019 08:30

History, 04.08.2019 08:30

Physics, 04.08.2019 08:30



Biology, 04.08.2019 08:30