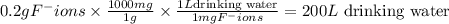Chemistry, 14.10.2019 23:30 anastasiakonni1
Fluoride ion is poisonous in relatively low amounts: 0.2 g of f− per 70 kg of body weight can cause death. nevertheless, in order to prevent tooth decay, f− ions are added to drinking water at a concentration of 1 mg of f− ion per l of water.
how many liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level?
answer in scientific notation

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Fluoride ion is poisonous in relatively low amounts: 0.2 g of f− per 70 kg of body weight can cause...
Questions

Physics, 10.03.2021 17:50

Mathematics, 10.03.2021 17:50



Mathematics, 10.03.2021 17:50



English, 10.03.2021 17:50




Mathematics, 10.03.2021 17:50

Mathematics, 10.03.2021 17:50

Mathematics, 10.03.2021 17:50

Mathematics, 10.03.2021 17:50

Mathematics, 10.03.2021 17:50




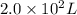 liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level
liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level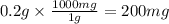 is toxic for 70kg person
is toxic for 70kg person