
Chemistry, 14.10.2019 22:10 foriegnngal
At 900 k the following reaction has kp=0.345: 2so2(g)+o2(g)ââ2so3(g) in an equilibrium mixture the partial pressures of so2 and o2 are 0.125 atm and 0.470 atm , respectively.
what is the equilibrium partial pressure of so3 in the mixture?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
At 900 k the following reaction has kp=0.345: 2so2(g)+o2(g)ââ2so3(g) in an equilibrium mixture the...
Questions



Mathematics, 20.01.2021 20:50



Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50

Social Studies, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

Biology, 20.01.2021 20:50


Mathematics, 20.01.2021 20:50

Biology, 20.01.2021 20:50


Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

Social Studies, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

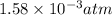
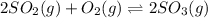 The expression for equilibrium constant for this reaction will be,
The expression for equilibrium constant for this reaction will be,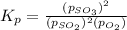
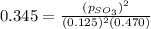
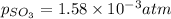
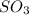 in the mixture is
in the mixture is 

