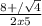Chemistry, 14.10.2019 21:00 missdee6929
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00 l container and the system is allowed to reach equilibrium. calculate the concentration of hi at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00...
Questions

Computers and Technology, 25.04.2021 19:30

Social Studies, 25.04.2021 19:30

English, 25.04.2021 19:30

Mathematics, 25.04.2021 19:30

Mathematics, 25.04.2021 19:30


Social Studies, 25.04.2021 19:30


Mathematics, 25.04.2021 19:40





Mathematics, 25.04.2021 19:40

Physics, 25.04.2021 19:40

Advanced Placement (AP), 25.04.2021 19:40

Chemistry, 25.04.2021 19:40


Mathematics, 25.04.2021 19:40

Mathematics, 25.04.2021 19:40

![Kc = \frac{{D}^dx[C]^c}{[A]^ax[B]^b}](/tpl/images/0319/5603/47fdd.png)
![Kc = \frac{[HI]^2}{[H2]x[I2]}](/tpl/images/0319/5603/fe065.png)