
Chemistry, 14.10.2019 17:30 alexthebest3976
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation? a. mg(s) + c(s) + 3/2 o_2(g) rightarrow mgco_3(s) b. c(s) + 1/2 o_2(g) rightarrow co(g) c. n_2(g) + o_2(g) rightarrow 2 no(g) d. n_2(g) + 2 o_2(g) rightarrow n_2o_4(g) e. h_2(g)+l/2 o_2(g) rightarrow h_2o(l)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 12:00
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1

Chemistry, 23.06.2019 15:30
The gas in a sealed container has an absolute pressure of 9.25 atmospheres. if the air around the container is at standard pressure, what is the gauge pressure inside the container
Answers: 1

Chemistry, 23.06.2019 17:00
Liquid nitrogen is kept at a temperature of -320 degrees. when liquid nitrogen is heated it quickly boils and turns into gas. which pair of pictures represent the change caused by adding heat to liquid nitrogen?
Answers: 3
You know the right answer?
Which of the following chemical equations does not correspond to a standard molar enthalpy of format...
Questions



Mathematics, 19.07.2021 19:10


Arts, 19.07.2021 19:10















 + O₂
+ O₂ → 2NO
→ 2NO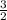 O
O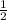 O₂
O₂


