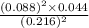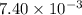Chemistry, 12.10.2019 03:10 shradhwaip2426
At a certain temperature, 0.760 mol so3 is placed in a 2.50 l container. 2so3(g)↽−−⇀2so2(g)+o2(g) at equilibrium, 0.110 mol o2 is present. calculate kc.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
At a certain temperature, 0.760 mol so3 is placed in a 2.50 l container. 2so3(g)↽−−⇀2so2(g)+o2(g) at...
Questions


Mathematics, 09.04.2021 01:00



Mathematics, 09.04.2021 01:00

Chemistry, 09.04.2021 01:00


Biology, 09.04.2021 01:00






English, 09.04.2021 01:00


Mathematics, 09.04.2021 01:00




Mathematics, 09.04.2021 01:00

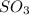 = 0.760 mol
= 0.760 mol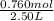
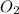 present at equilibrium = 0.110 mol
present at equilibrium = 0.110 mol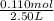
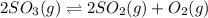
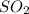 = 2x
= 2x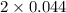
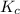 ) as follows.
) as follows.![K_{c} = \frac{[SO_{2}]^{2}[O_{2}]}{[SO_{3}]^{2}}](/tpl/images/0312/0764/e07bc.png)