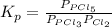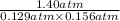Chemistry, 11.10.2019 23:30 sissygirl0807
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl₃(g)+cl₂(g)⇌pcl₅(g). a 7.5-l gas vessel is charged with a mixture of pcl₃(g) and cl₂(g), which is allowed to equilibrate at 450 k. at equilibrium the partial pressures of the three gases are ppcl₃ = 0.129 atm, pcl₂ = 0.156 atm, and ppcl₅ = 1.40 atm. what is the value of kp at this temperature? express your answer using three significant figures.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl₃(g)+cl₂...
Questions














Mathematics, 22.09.2019 15:10

History, 22.09.2019 15:10


Computers and Technology, 22.09.2019 15:10


History, 22.09.2019 15:10

Social Studies, 22.09.2019 15:10

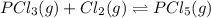
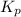 will be as follows.
will be as follows.