
Chemistry, 11.10.2019 22:30 tahiratnoel20
using only the information available in the periodic table, consider the elements potassium and bromine. from their location on the periodic table, identify the oxidation state and number of valence electrons for potassium and bromine. then use this information to describe their reactivity.
which statement most accurately describes the compound formed by potassium and bromine?
a) potassium and bromide form an ionic compound called potassium bromide (kbr).
b) potassium and bromide form an ionic compound called potassium bromide (k2br).
c) potassium and bromide share electrons to form a covalent compound called potassium monobromide (kbr).
d) potassium and bromide share electrons to form a covalent compound called potassium dibromide (kbr2).
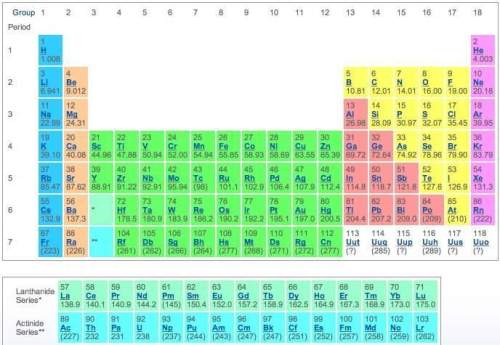

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
using only the information available in the periodic table, consider the elements potassium and brom...
Questions

Mathematics, 22.01.2021 17:00

Mathematics, 22.01.2021 17:00

Biology, 22.01.2021 17:00

Mathematics, 22.01.2021 17:00



Mathematics, 22.01.2021 17:00



Chemistry, 22.01.2021 17:00


Social Studies, 22.01.2021 17:00

Mathematics, 22.01.2021 17:00

English, 22.01.2021 17:00


Mathematics, 22.01.2021 17:00


Mathematics, 22.01.2021 17:00

Mathematics, 22.01.2021 17:00

English, 22.01.2021 17:00



