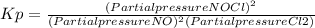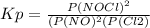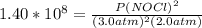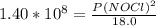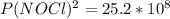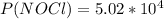Chemistry, 11.10.2019 17:30 BatmanVS1944
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no and 2.0 atm of cl2(g). what is the pressure of no(g) when equilibrium is reached?
3.6 × 10-4 atm
3.8 × 10-8 atm
0.5 atm
2.0 atm
1.0 atm
1.1 × 10-7 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no...
Questions


Mathematics, 27.06.2019 22:00

English, 27.06.2019 22:00



History, 27.06.2019 22:00


Health, 27.06.2019 22:00




Business, 27.06.2019 22:00




Physics, 27.06.2019 22:00


History, 27.06.2019 22:00

Biology, 27.06.2019 22:00