
Chemistry, 11.10.2019 03:30 mdndndndj7365
Consider the following reaction and select the false statement below.
10 i−(aq) + 16 h+(aq) + 2 mno4−(aq) → 5 i2(s) + 2 mn2+(aq) + 8 h2o(l)
(a) this is a reduction-oxidation (redox) reaction
(b) the iodine ion is the reducing agent
(c) the permanganate ion is the oxidizing agent
(d) the hydrogen ion is neither reduced nor oxidized
(e) in the reaction as written, ten moles of electrons are transferred from the oxidizing agent to the reducing agent

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Consider the following reaction and select the false statement below.
10 i−(aq) + 16 h+(aq) +...
10 i−(aq) + 16 h+(aq) +...
Questions

Mathematics, 28.04.2021 22:10

Mathematics, 28.04.2021 22:10

Mathematics, 28.04.2021 22:10

Mathematics, 28.04.2021 22:10

English, 28.04.2021 22:10


Chemistry, 28.04.2021 22:10

Arts, 28.04.2021 22:10


Mathematics, 28.04.2021 22:10

Mathematics, 28.04.2021 22:10



Computers and Technology, 28.04.2021 22:10

History, 28.04.2021 22:10

Mathematics, 28.04.2021 22:10



Chemistry, 28.04.2021 22:10

History, 28.04.2021 22:10

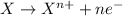
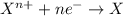
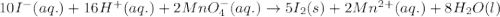
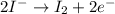 ( × 5)
( × 5)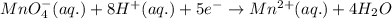 ( × 2)
( × 2)

