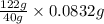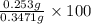An unknown amount of acid can often be determined by adding an excess of base and then back-titrating the excess. a 0.3471−g sample of a mixture of oxalic acid, which has two ionizable protons, and benzoic acid, which has one, is treated with 97.0 ml of 0.1090 m naoh. the excess naoh is titrated with 21.00 ml of 0.2060 m hcl. find the mass % of benzoic acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1


Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
An unknown amount of acid can often be determined by adding an excess of base and then back-titratin...
Questions


Mathematics, 29.08.2019 19:30

Social Studies, 29.08.2019 19:30

Arts, 29.08.2019 19:30


Health, 29.08.2019 19:30

History, 29.08.2019 19:30

Mathematics, 29.08.2019 19:30



Physics, 29.08.2019 19:30

History, 29.08.2019 19:30

English, 29.08.2019 19:30



Mathematics, 29.08.2019 19:30



Mathematics, 29.08.2019 19:30

Advanced Placement (AP), 29.08.2019 19:30

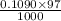
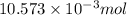
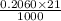
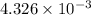 mol
mol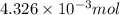
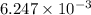 mol
mol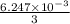
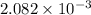 mol
mol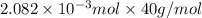
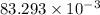 g
g