
Chemistry, 11.10.2019 03:00 Rainey1664
Naoh was titrated to the phenolphthalein endpoint with a known concentration of hcl. the student conducting the experiment placed 12.97 ml of 2.74 m hcl in a beaker with the indicator. before beginning the reaction, he noted that the buret read a volume of 4.50 ml. after reaching the endpoint, the buret read 13.65 ml. calculate the molarity of naoh that reacted upon reaching the endpoint. be sure to report your answer to the appropriate number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Naoh was titrated to the phenolphthalein endpoint with a known concentration of hcl. the student con...
Questions

Mathematics, 20.04.2021 19:30

English, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30



Chemistry, 20.04.2021 19:30

English, 20.04.2021 19:30

World Languages, 20.04.2021 19:30


Mathematics, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30


Mathematics, 20.04.2021 19:30

English, 20.04.2021 19:30





Mathematics, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30

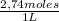 = 0,0347 moles HCl ≡ 0,0347 moles NaOH
= 0,0347 moles HCl ≡ 0,0347 moles NaOH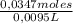 = 3,65M
= 3,65M

