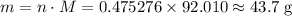50.0 g of n2o4(g) is placed in a 2.0 l evacuated flask and the system is allowed to reach equilibrium according to the reaction: n2o4(g) ⇄ 2 no2(g) kc = 0.133 after the system reaches equilibrium, 5.00 g of no2(g) is injected into the vessel, and the system is allowed to equilibrate once again. calculate the mass of n2o4 in the final equilibrium mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
50.0 g of n2o4(g) is placed in a 2.0 l evacuated flask and the system is allowed to reach equilibriu...
Questions

Mathematics, 12.05.2021 04:50

Mathematics, 12.05.2021 04:50


Mathematics, 12.05.2021 04:50


History, 12.05.2021 04:50


History, 12.05.2021 04:50

Social Studies, 12.05.2021 04:50









Social Studies, 12.05.2021 04:50


Chemistry, 12.05.2021 04:50

 .
.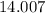 ;O:
;O: 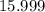 .
.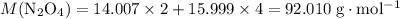 .
.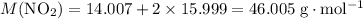 .
.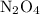 in that sample of
in that sample of 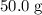 :
: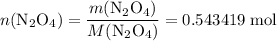 .
. in that
in that 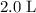 container:
container: 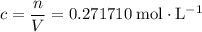 .
.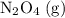 be
be 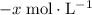 .
.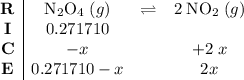 .
.![\displaystyle \frac{[\rm NO_2]^{2}}{[\rm N_2O_4]} = \rm K_{c} = 0.133](/tpl/images/0308/9684/7a7f2.png) .
.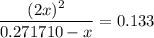 .
. .
. 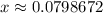 .
.![\rm [N_2O_4] \approx 0.271710 - 0.0798672 = \rm 0.191843 \;mol\cdot L^{-1}](/tpl/images/0308/9684/a5112.png) .
.![\rm [NO_2] \approx 2 \times 0.0798672 = 0.159734\; mol\cdot L^{-1}](/tpl/images/0308/9684/f286e.png) .
.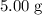 of
of ![\rm[NO_2]](/tpl/images/0308/9684/e223a.png) if it was added to an evacuated
if it was added to an evacuated 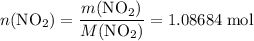 .
.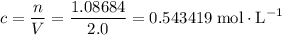 .
.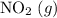 will become
will become 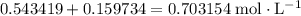 .
.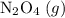 be
be 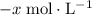 .
.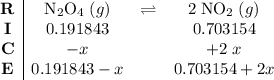 .
. 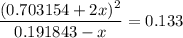 .
.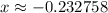 .
.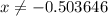 for if that would lead to a negative value for the concentration of
for if that would lead to a negative value for the concentration of 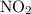 .
.![[{\rm N_2O_4}] = 0.703154 + 2(-0.232758) = \rm 0.237638\; mol\cdot L^{-1}](/tpl/images/0308/9684/8370c.png) .
.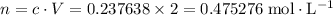 .
.