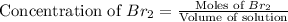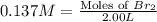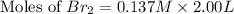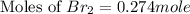Chemistry, 11.10.2019 03:00 simiyi1983
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen bromide: h2 (g) + br2 (g) ↔ 2hbr (g) a mixture of 0.682 mol of h2 and 0.440 mol of br2 is combined in a reaction vessel with a volume of 2.00 l. at equilibrium at 700 k, there are 0.516 mol of h2 present. at equilibrium, there are mol of br2 present in the reaction vessel.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen...
Questions


Mathematics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00


Arts, 02.01.2021 14:00



Chemistry, 02.01.2021 14:00


Health, 02.01.2021 14:00



Chemistry, 02.01.2021 14:00

English, 02.01.2021 14:00

History, 02.01.2021 14:00

Chemistry, 02.01.2021 14:00


Social Studies, 02.01.2021 14:00

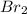 at equilibrium is 0.274 mole.
at equilibrium is 0.274 mole.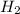 and
and 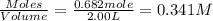
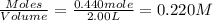
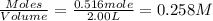
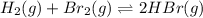
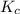 will be,
will be,![K_c=\frac{[HBr]^2}{[H_2][Br_2]}](/tpl/images/0308/9786/3b8cb.png)