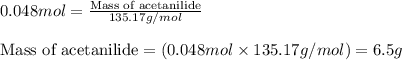Chemistry, 11.10.2019 02:20 roneesmith2016
The expression of the theoretical yield (ty) in function of limiting reagent (lr) of a reaction is as follows: ty = ideal mole ratio of (target product / lr) x #mol(lr) x mw(target product) the ideal mole ratio is the one provided by the equation of the reaction. if a reaction uses (4.50x10^0) g of aniline and 1.25 times as many ml of acetic anhydride as the number of grams of aniline, what is the theoreticl yiled of acetanilide (mw = 135.17 g/mol) in the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
The expression of the theoretical yield (ty) in function of limiting reagent (lr) of a reaction is a...
Questions





Mathematics, 08.03.2021 03:50


Mathematics, 08.03.2021 03:50

Law, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50

Advanced Placement (AP), 08.03.2021 03:50

Mathematics, 08.03.2021 03:50






Mathematics, 08.03.2021 03:50


English, 08.03.2021 03:50

English, 08.03.2021 03:50

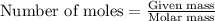 .....(1)
.....(1)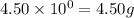 (We know that:
(We know that:  )
)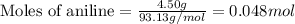
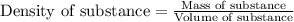
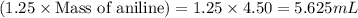
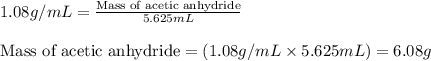
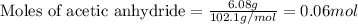
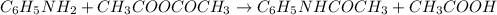
 of acetic anhydride
of acetic anhydride