
Chemistry, 10.10.2019 02:00 thetudent41
Calculate the number of moles of al2o3 that are produced when 0.60 mol of fe is produced in the following reaction: 2al(s)+3feo(s) = 3fe(s)+al2o3(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1


Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Calculate the number of moles of al2o3 that are produced when 0.60 mol of fe is produced in the foll...
Questions



History, 04.02.2020 06:56

Chemistry, 04.02.2020 06:56


Mathematics, 04.02.2020 06:56

History, 04.02.2020 06:56

English, 04.02.2020 06:56


Social Studies, 04.02.2020 06:56

Social Studies, 04.02.2020 06:56




Geography, 04.02.2020 06:56


Physics, 04.02.2020 06:56


Mathematics, 04.02.2020 06:56

Physics, 04.02.2020 06:56

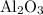 produced is
produced is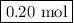 .
.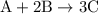
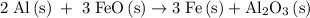
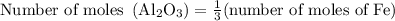 …… (1)
…… (1)
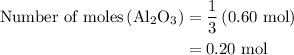
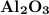 is produced when 0.60 mol of Fe is produced in the reaction.
is produced when 0.60 mol of Fe is produced in the reaction.

