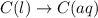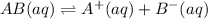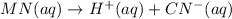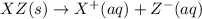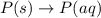Chemistry, 11.10.2019 00:10 look26goingjbgy
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. c(l)→c(aq) ab(aq)⇌ a+(aq)+b−(aq) mn(aq)→m+(aq)+n−(aq) xz(s)→x+(aq)+z−(aq) p(s)→p(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong...
Questions

English, 31.01.2020 14:53


History, 31.01.2020 14:53




Health, 31.01.2020 14:53


Chemistry, 31.01.2020 14:53


Spanish, 31.01.2020 14:53



Mathematics, 31.01.2020 14:53


History, 31.01.2020 14:53



Geography, 31.01.2020 14:53

Biology, 31.01.2020 14:53