
Chemistry, 11.10.2019 00:00 jackfrost5
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.0020 m kscn . the standard solution had an absorbance of 0.480 . fe3+(aq)+scn−(aq)↽−−⇀fescn2+(aq) a trial solution was made in a similar manner, but with a more dilute fe(no3)3 reagent. the initial scn− concentration, immediately after mixing, was 0.00050 m . this trial solution had absorbance of 0.220 . what is the equilibrium concentration of scn− in the trial solution?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.00...
Questions

Mathematics, 10.03.2021 01:20



Mathematics, 10.03.2021 01:20

Mathematics, 10.03.2021 01:20

Chemistry, 10.03.2021 01:20

Mathematics, 10.03.2021 01:20


Mathematics, 10.03.2021 01:20

Mathematics, 10.03.2021 01:20




Mathematics, 10.03.2021 01:20




History, 10.03.2021 01:20


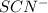 in the trial solution is
in the trial solution is 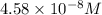
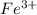 and
and 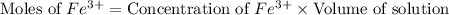
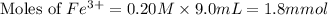
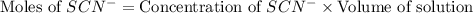
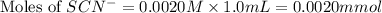
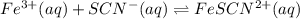
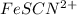
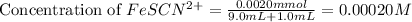

 = molar absorptivity coefficient
= molar absorptivity coefficient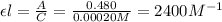
![[SCN^-]_{eqm}=[SCN^-]_{initial}-[FeSCN^{2+}]](/tpl/images/0308/4929/92d10.png)
![[SCN^-]_{initial}](/tpl/images/0308/4929/bc58d.png) = 0.00050 M
= 0.00050 M![[FeSCN^{2+}]](/tpl/images/0308/4929/797d4.png) .
.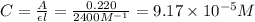
![[SCN^-]_{eqm}=(0.00050M)-(9.17\times 10^{-5}M)](/tpl/images/0308/4929/c2881.png)
![[SCN^-]_{eqm}=4.58\times 10^{-8}M](/tpl/images/0308/4929/1e6f2.png)


