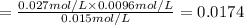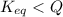At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes place in a 10.0 l vessel at 1173 k. if a mixture of 15.0 g caco3, 15.0 g cao, and 4.25 g co2 is allowed to approach equilibrium, what will happen to the amount of caco3? group of answer choices
- it will remain the same
- it will increase
- not enough information is provided to answer this question
- it will decrease

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 10:30
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3
You know the right answer?
At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes p...
Questions

Mathematics, 17.09.2019 16:30

Social Studies, 17.09.2019 16:30


Social Studies, 17.09.2019 16:30




Business, 17.09.2019 16:30










Mathematics, 17.09.2019 16:30

Mathematics, 17.09.2019 16:30

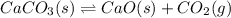
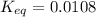
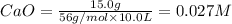
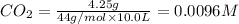
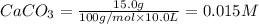
![Q=\frac{[CaO][CO_2]}{[CaCO_3]}](/tpl/images/0308/1118/76f7b.png)