
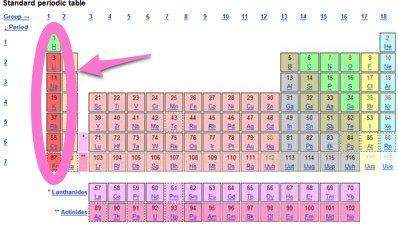
As you move down through the family of alkali metals, the first ionization energy because
a) increases; the atomic radii increase.
b) increases; the atomic radii decrease.
c) decreases; the atomic radii increase.
d) remains constant; all the elements lose one electron.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
As you move down through the family of alkali metals, the first ionization energy because
Questions

Chemistry, 17.10.2019 16:50

History, 17.10.2019 16:50

Mathematics, 17.10.2019 16:50


Biology, 17.10.2019 16:50



Mathematics, 17.10.2019 16:50




Geography, 17.10.2019 16:50

Social Studies, 17.10.2019 16:50



English, 17.10.2019 16:50

Mathematics, 17.10.2019 16:50


Health, 17.10.2019 16:50




